Abstract
BACKGROUND: IDH2 mutations occur in 8-19% of patients (pts) with AML. ENA is an oral, selective IDH2 inhibitor and AZA is a hypomethylating agent. In a phase 1b/2 trial, combination therapy with ENA + SC AZA significantly improved overall response rate (ORR) and complete remission (CR) rate vs AZA monotherapy (AZA-only) (P = 0.0064 and P = 0.0001, respectively) in pts with newly diagnosed (ND), mutant-IDH2 (m IDH2) AML not eligible for IC (DiNardo, 2020). Injectable AZA has shown to maintain pt-reported health-related quality of life (HRQoL) in ND-AML (Dombret, 2014); the effect of ENA on HRQoL has not been reported. While morphologic responses are associated with improved HRQoL, combining active agents could increase toxicity, which could diminish HRQoL.
OBJECTIVE: Assess pt-reported HRQoL outcomes during treatment (Tx) with ENA + AZA and AZA-only in the phase 1b/2 AG-221-AML-005 trial (NCT02677922).
METHODS: Adult pts with ECOG PS ≤ 2 and intermediate- or poor-risk cytogenetics were enrolled. In the phase 2 portion, pts were randomized 2:1 to ENA 100-mg QD (continuous) + AZA 75 mg/m 2/day (d) SC × 7d, or to AZA-only, in repeated 28d cycles (C). Pt-reported HRQoL was assessed during Tx using the EORTC QLQ-C30 and EQ-5D-5L instruments, each completed at baseline (BL [C1D1]), on d1 of each Tx cycle, and end of Tx (EOT). The primary HRQoL measures were observed mean changes from BL (CFB) in 5 QLQ-C30 domains-Global Health status (GHS)/QoL, Physical Functioning (PF), Role Functioning (RF), Fatigue, and Dyspnea-each scored from 0-100, with higher scores indicating better QoL or functioning but worse symptomology. Changes in the remaining 10 QLQ-C30 domains, and in the EQ-5D-5L utility index (UI) score (derived via cross-walk to EQ-5D-3L based on UK population weights) and EQ-5D visual analogue scale (VAS) score (0-100) were secondary outcomes. Changes are reported for Tx cycles with ≥ 10 evaluable pts in each Tx arm. Clinically meaningful changes were defined as mean CFB of ≥ 10 points on any QLQ-C30 domain, ≥ 0.08 point on the EQ-5D-5L UI, and ≥ 7 points on the EQ-5D VAS. HRQoL-evaluable pts had an evaluable assessment (≥ 15 items on the QLQ-C30; all 5 EQ-5D-5L items; non-missing EQ-5D VAS) at BL and at ≥ 1 post-BL visit.
RESULTS: The HRQoL-evaluable population comprised ~75% of pts randomized to either ENA+AZA (51/68) or AZA-only (25/33); 25 pts were not evaluable due to missing data at BL (n = 16) and/or no post-BL visit (n = 19). QLQ-C30 data were missing for ~20%-30% of eligible pts at almost all post-BL visits through C9D1 (the last visit with ≥ 10 pts in each Tx arm), and for ≥ 50% of pts at the EOT visit; similar rates were observed on the EQ-5D-5L. BL characteristics for HRQoL-evaluable pts were generally comparable between arms and similar to those of the ITT population. At BL, mean QLQ-C30 scores in each Tx arm were meaningfully worse than general population normative scores across the majority of QLQ-C30 domains, including all 5 domains of primary interest, and on the EQ-5D VAS.
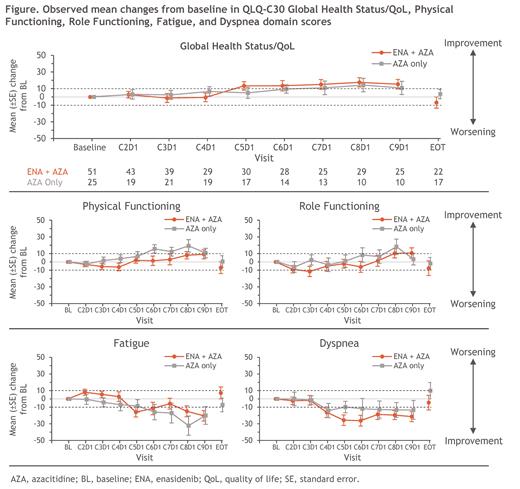
During early ENA+AZA Tx cycles, observed mean QLQ-C30 scores remained similar to BL in the GHS/QoL and Dyspnea domains, and were worsened from BL in the PF, RF, and Fatigue domains-but reached the threshold for meaningful worsening only in the RF domain and only at C3D1 (Figure). Domain scores then trended toward improvement over time, with clinically meaningful improvements from BL from C4-C9 in the Dyspnea domain, C5-C9 in the GHS/QoL and Fatigue domains (except at C7 for Fatigue), and from C8-C9 in the RF domain; mean scores also improved from BL in the PF domain but were not clinically meaningful at any visit. Similar trends were observed in the AZA-only arm but without the early worsening seen with ENA+AZA (Figure). There were no clinically meaningful or statistically significant differences between Tx arms in mean CFB for any of the primary QLQ-C30 domains through C9. In both Tx arms, mean score CFB in the secondary QLQ-C30 domains, EQ-5D-5L UI, and EQ-5D VAS followed similar trends of improvement over time among pts who remained on Tx.
CONCLUSIONS: At BL, these pts with AML reported meaningfully worse HRQoL scores across the majority of the QLQ-C30 domains and the EQ-5D VAS compared with the general population, indicating substantial impairment in HRQoL, functioning, and symptoms. HRQoL scores generally worsened during early ENA+AZA Tx cycles, but then improved with continued Tx, with meaningful improvement across multiple measures during later Tx cycles.
DiNardo: Agios/Servier: Consultancy, Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Forma: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding; Novartis: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Takeda: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding. Dohner: Pfizer: Research Funding; Roche: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; GEMoaB: Honoraria; Janssen: Honoraria; Helsinn: Honoraria; Gilead: Honoraria; Berlin-Chemie: Honoraria; Astex Pharmaceuticals: Honoraria; Astellas: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AstraZeneca: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Oxford Biomedica: Honoraria; Abbvie: Honoraria, Research Funding. Zeidan: Agios: Consultancy; AstraZeneca: Consultancy; BioCryst: Other: Clinical Trial Committees; Daiichi Sankyo: Consultancy; Genentech: Consultancy; Cardiff Oncology: Consultancy, Other: Travel support, Research Funding; Incyte: Consultancy, Research Funding; Janssen: Consultancy; Novartis: Consultancy, Other: Clinical Trial Committees, Travel support, Research Funding; Pfizer: Other: Travel support, Research Funding; Amgen: Consultancy, Research Funding; Ionis: Consultancy; Gilead: Consultancy, Other: Clinical Trial Committees; Astellas: Consultancy; BMS: Consultancy, Other: Clinical Trial Committees, Research Funding; Geron: Other: Clinical Trial Committees; Acceleron: Consultancy, Research Funding; Loxo Oncology: Consultancy, Other: Clinical Trial Committees; Kura: Consultancy, Other: Clinical Trial Committees; Boehringer Ingelheim: Consultancy, Research Funding; Aprea: Consultancy, Research Funding; Jasper: Consultancy; Epizyme: Consultancy; Jazz: Consultancy; ADC Therapeutics: Research Funding; BeyondSpring: Consultancy; Astex: Research Funding; AbbVie: Consultancy, Other: Clinical Trial Committees, Research Funding. Schuh: Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlycoMimetics: Research Funding; Kite/Gilead: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Teva: Honoraria, Membership on an entity's Board of Directors or advisory committees. Vyas: Gilead: Honoraria; Jazz: Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Takeda: Honoraria; Astellas: Consultancy, Honoraria; Pfizer: Honoraria; Daiichi Sankyo: Honoraria; Janssen: Honoraria; AbbVie: Consultancy, Honoraria. Stein: Janssen Pharmaceuticals: Consultancy; Abbvie: Consultancy; Gilead Sciences, Inc.: Consultancy; Blueprint Medicines: Consultancy; Foghorn Therapeutics: Consultancy; Jazz Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; Celgene: Consultancy; PinotBio: Consultancy; Daiichi Sankyo: Consultancy; Syros Pharmaceuticals, Inc.: Consultancy; Genentech: Consultancy; Syndax Pharmaceuticals: Consultancy; Agios Pharmaceuticals, Inc: Consultancy; Astellas: Consultancy; Novartis: Consultancy. Wei: Novartis, Abbvie, Celgene/BMS: Speakers Bureau; Abbvie, Amgen, Astellas, AstraZeneca, Celgene/BMS, Genentech, Janssen, MacroGenics, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees; Former employee of Walter and Eliza Hall Institute: Patents & Royalties: Prof. Andrew Wei is a former employee of the Walter and Eliza Hall Institute and is eligible for a fraction of the royalty stream related to Venetoclax; Abbvie, Amgen, AstraZeneca, Celgene/BMS, Novartis, Servier and F. Hoffmann-La Roche: Research Funding; Abbvie, Amgen, Astellas, AstraZeneca, Celgene/BMS, Genentech, Janssen, MacroGenics, Novartis, Pfizer, and Servier: Honoraria; Servier: Consultancy. de Botton: Celgene: Consultancy; Agios: Consultancy, Honoraria; Forma: Consultancy, Honoraria; Astellas: Consultancy; Abbvie: Consultancy; Daiichi: Consultancy; Novartis: Consultancy; Jazz: Consultancy; Pfizer: Consultancy. Chen: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Lord-Bessen: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Martin-Regueira: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Lersch: Celgene, a Bristol-Myers Squibb Company: Current Employment. Gong: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Guo: Bristol Myers Squibb: Consultancy; Daiichi Sankyo: Consultancy; UCB: Consultancy; Janssen: Consultancy; Gilead: Consultancy; EMD Serono: Consultancy; Evidera: Current Employment. Shi: Bristol Myers Squibb: Consultancy. Montesinos: Teva: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Stemline/Menarini: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Forma Therapeutics: Consultancy; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Glycomimetics: Consultancy; Tolero Pharmaceutical: Consultancy; Agios: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas Pharma, Inc.: Consultancy, Honoraria, Other: Advisory board, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal